1xbet is a popular bookmaker offering its clients a wide range of sports betting and gambling. In order to start playing and betting on the 1xbet website, you must complete the registration procedure. However, the company offers its users a simplified one-click registration form that allows you to quickly and easily create an account on […]
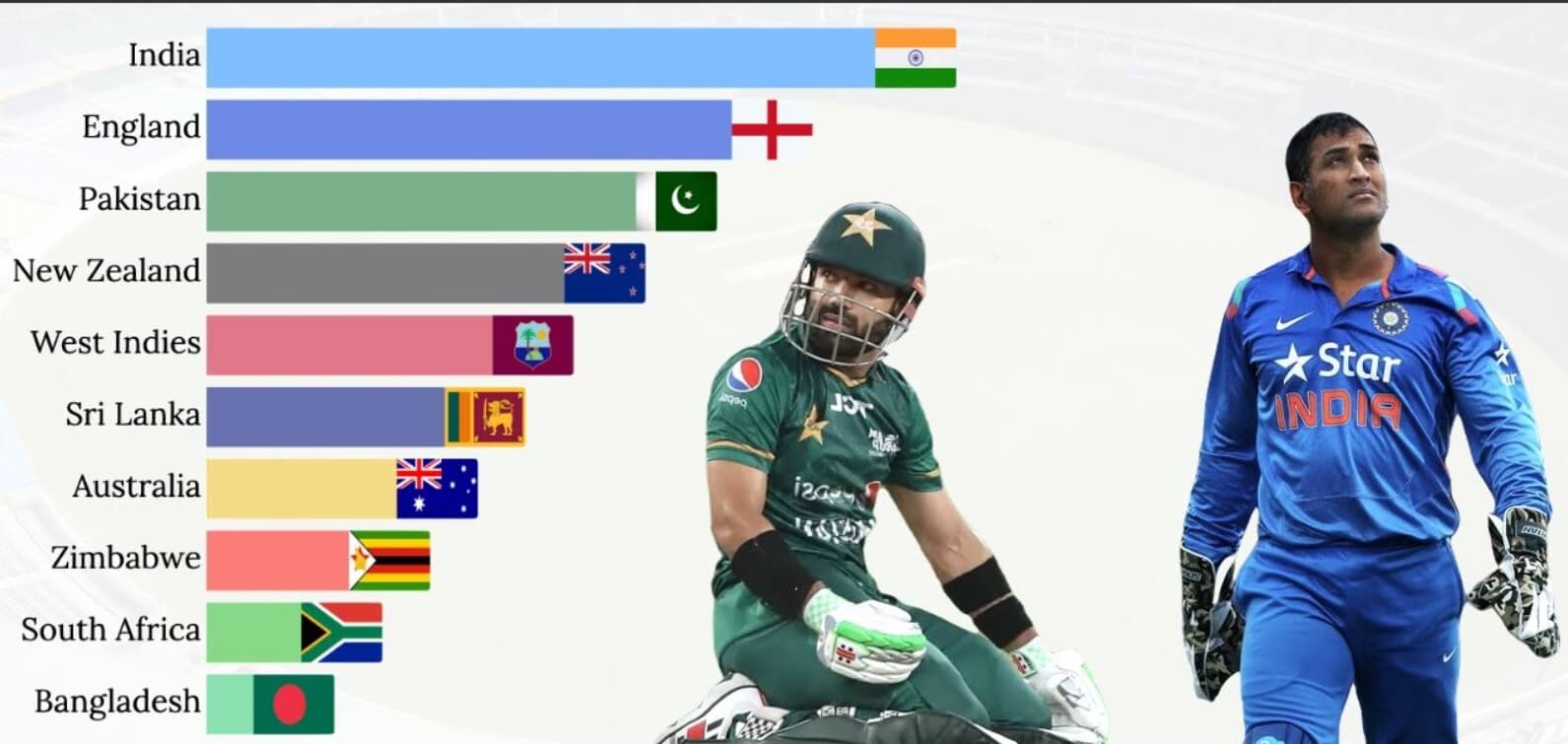
Cricket, a sport with a rich history and global following, hinges on statistics, and among these, the average stands out as a crucial measure of a player’s skill and consistency. In cricket, averages are used to gauge the performance of batsmen and bowlers – for batsmen, it’s the number of runs scored per dismissal, while […]
Having access to your favorite sports markets and casino games on the go is essential. You need to 1xBet download this will allow you to enjoy a seamless betting experience from your mobile device. This step-by-step guide will walk you through the process of downloading and installing the 1xBet app, ensuring that you can make the most of your betting adventure, anytime and anywhere.
Welcome to the thrilling world of sports betting on 1xBet, a platform that offers an extensive array of options for both novice and experienced bettors. In this comprehensive guide, we will delve into the realm of sports betting on 1xBet, exploring various facets of the platform, from the diverse sports markets to the intricate betting […]
Finding a legitimate and safe betting site is crucial for a secure and enjoyable gambling experience. One name that has gained significant attention in recent years is 1xBet. But the big question remains: Is 1xBet a legitimate and safe betting site? In this comprehensive article, we will explore every aspect of 1xBet, providing insights based […]
The world of online betting has witnessed a remarkable transformation over the years, with numerous platforms vying for the attention of eager bettors. One such prominent player in this field is 1xBet. In this comprehensive article, we’ll take you on an enlightening journey through the realm of online betting platforms, focusing on 1xBet and comparing […]
Welcome to the exciting world of online sports betting, where opportunities to turn your sports knowledge into real winnings await you. In this beginner’s guide, we will delve into the intricacies of using 1xBet, one of the leading platforms in the industry. Whether you’re new to sports betting or looking to switch to a more […]
At its core, 1xBet is an online betting platform that allows users to bet on a wide range of sports, casino games, and more. It’s a versatile and user-friendly platform that caters to both beginners and experienced bettors. With a vast selection of betting options and an intuitive interface, 1xBet has become a go-to choice […]